38 fda health claims on food labels
CFR - Code of Federal Regulations Title 21 - Food and Drug Administration (1) health claim means any claim made on the label or in labeling of a food, including a dietary supplement, that expressly or by implication, including "third party" references, written statements... Label Claims for Conventional Foods and Dietary Supplements there are three ways in which fda exercises its oversight in determining which health claims may be used on a label or in labeling for a conventional food or dietary supplement: 1) the 1990...
eCFR :: 21 CFR Part 101 -- Food Labeling § 101.1 Principal display panel of package form food. The term principal display panel as it applies to food in package form and as used in this part, means the part of a label that is most likely to be displayed, presented, shown, or examined under customary conditions of display for retail sale. The principal display panel shall be large enough to accommodate all the mandatory label ...

Fda health claims on food labels
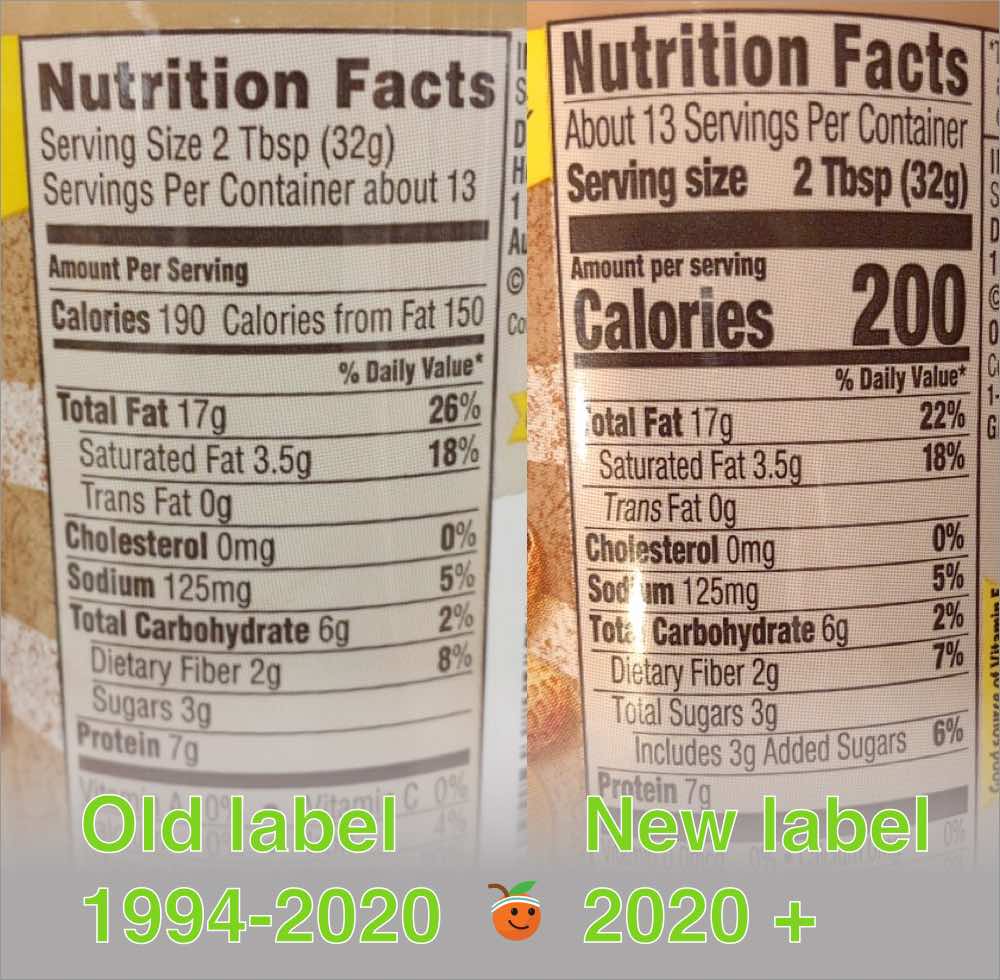
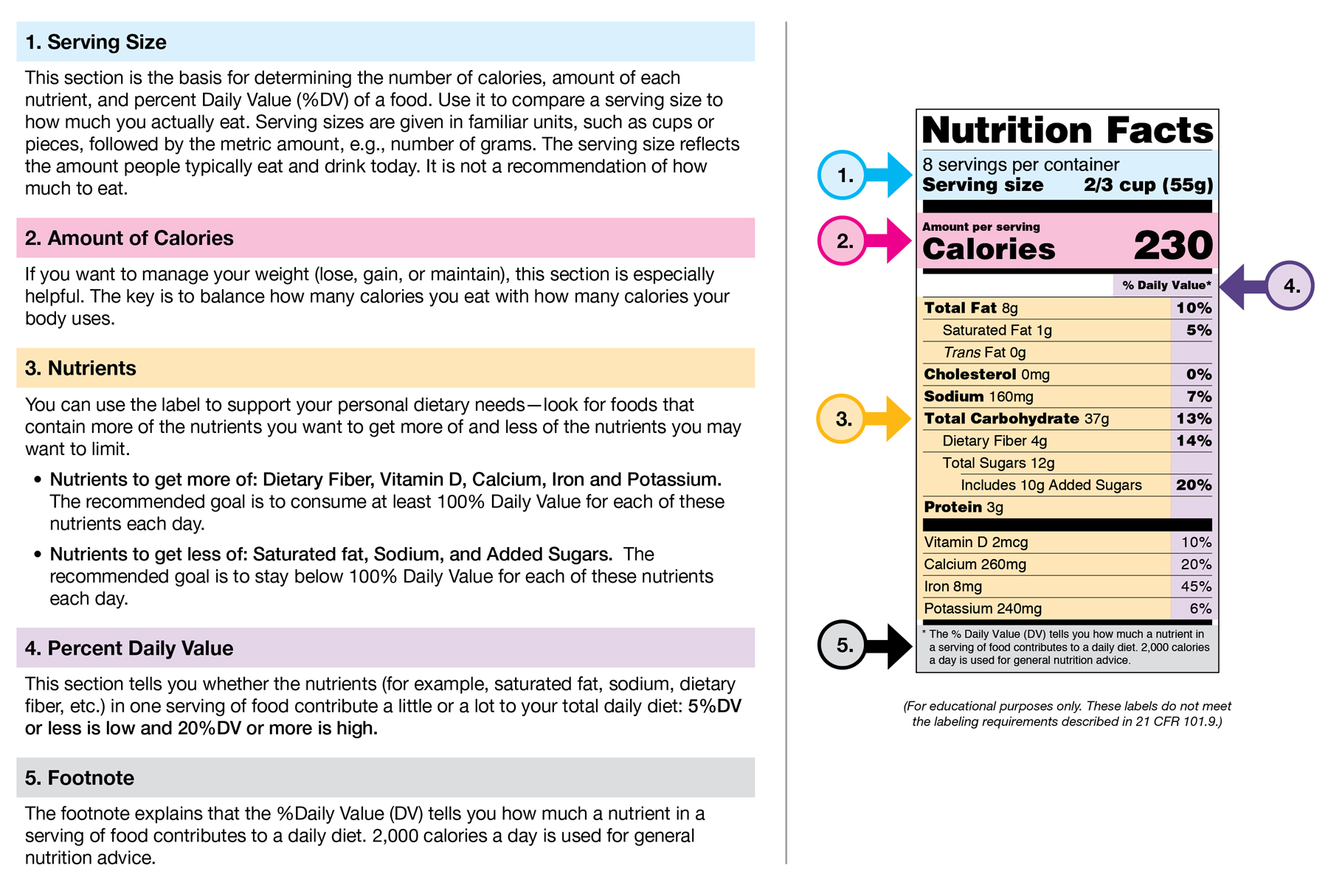
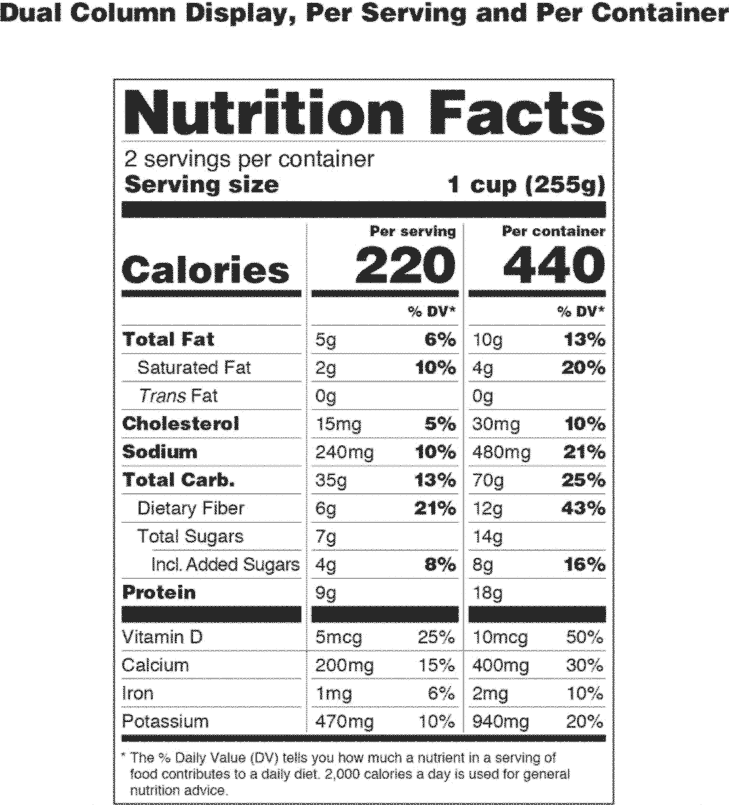
Food Packaging Claims | American Heart Association It's important to understand what these claims mean so you can make informed decisions about the food you buy for yourself and your family. There are three categories of claims defined by statute and/or FDA regulations that can be used on food and dietary supplement labels: health claims, nutrient content claims, and structure/function claims. Qualified Health Claims - FDA Food manufacturers can petition the agency to consider exercising enforcement discretion for the use of a qualified health claim. The FDA does not "approve" qualified health claim petitions. Changes to the Nutrition Facts Label | FDA - U.S. Food and ... Mar 07, 2022 · Manufacturers with $10 million or more in annual sales were required to update their labels by January 1, 2020; manufacturers with less than $10 million in annual food sales were required to ...
Fda health claims on food labels. Advice about Eating Fish | FDA - U.S. Food and Drug ... Jun 08, 2022 · A healthy eating pattern consists of choices across all food groups (vegetables, fruits, grains, dairy, and protein foods, which includes fish), eaten in recommended amounts, and within calorie ... 5 Understanding Food Labels and Health Claims - Maricopa Health Claims & Foods To keep companies from making false claims, the FDA provides food manufacturers' regulations in putting labels on packages that promote health. There are three levels of health claims: A health claim is supported by scientific evidence. An example is "reduces heart disease." Guidance on Substantiation for Dietary Supplement Claims (1) The Office of Nutrition, Labeling, and Dietary Supplements in FDA's Center for Food Safety and Applied Nutrition prepared this guidance document. (2) Under section 403(r)(6)(A) of the Act (21 ... FDA Proposes Updated Definition of 'Healthy' Claim on Food Packages To ... Today, the U.S. Food and Drug Administration proposed updated criteria for when foods can be labeled with the nutrient content claim "healthy" on their packaging. This proposed rule would align the definition of the "healthy" claim with current nutrition science, the updated Nutrition Facts label and the current Dietary Guidelines for Americans.
Use of the Term Healthy on Food Labeling - FDA 6 days ago — Foods must meet specific nutrient-related criteria to use the nutrient content claim “healthy.” The FDA has begun a public process to update the ... Auburn food historian explains new FDA guidelines for 'healthy' food labels Last week, the U.S. Food and Drug Administration, or FDA, updated its criteria for foods labeled "healthy." The proposed change is based on current nutrition science and prioritizes healthy dietary patterns, continuing from the FDA's overhaul of the Nutrition Facts panel in 2016. Are the FDA's new 'healthy' food labels misleading? According to the U.S. Food and Drug Administration, foods must meet specific nutrient-related criteria to use the nutrient content that is claimed to be "healthy." The FDA has begun a process to update the "healthy" claim on food labeling to be consistent with current nutritional science and federal dietary guidelines. A Guide to FDA Regulation of Food Labeling Claims Among the FDA-regulated claims commonly declared on food labels are nutrient-content claims, health claims, qualified health claims and structure/function claims. Additionally, FDA has authority over claims related to gluten content, genetically modified organisms (GMOs) and "natural."
Questions and Answers on Health Claims in Food Labeling All health claims, whether authorized or qualified, require pre-market review by the FDA. Under federal law, the FDA approves by regulation authorized health claims for use in food labeling only if... Structure/Function Claims - FDA 7 Mar 2022 — If a dietary supplement label includes such a claim, it must state in a "disclaimer" that FDA has not evaluated the claim. The disclaimer must ... Misleading Nutrition and Food Labels - Health Jun 07, 2012 · 16 Most Misleading Food Labels ... If they make medical claims, it can trigger intense scrutiny from the FDA and the federal trade commission. In 2008, the company that makes the vitamin product ... FDA proposes updates to 'healthy' claim on food packages | CNN In order to be labeled with the "healthy" claim, products would need to: Contain a certain, meaningful amount of food from at least one of the food groups or subgroups - such as fruits, vegetables...
Label Claims for Food & Dietary Supplements | FDA Mar 07, 2022 · Among the claims that can be used on food and dietary supplement labels are three categories of claims that are defined by statute and/or FDA regulations: health claims, nutrient content claims ...
Introduction to Food Product Claims — FDA Reader A Qualified Health Claim is a statement approved by the FDA for use on food labels that has strict wording requirements. When there is emerging evidence between a food and the reduced risk of a disease or health condition, but not enough for the FDA to issue an Authorized Health Claim, the FDA may approve a "Qualified Health Claim".
FDA's plan to define 'healthy' for food packaging: Do we really need it? To illustrate the "healthy" claim, the FDA is also researching a symbol that food makers can use, and might be testing examples like these. Food and Drug Administration Doing all this, the FDA...
Health claims on food labels - PubMed Health claims on food labels Food and drug law requires that the ingredients in most foods be disclosed on their labels, but until recently there was no requirement that nutrition information be provided. The Nutrition Labeling and Education Act of 1990 (NLEA), passed on November 8, 1990, mandated the Food and Drug Administrati …
The Dangers of Raw Milk: Unpasteurized Milk Can Pose a ... Milk and milk products provide a wealth of nutrition benefits. But raw milk, i.e., unpasteurized milk, can harbor dangerous microorganisms that can pose serious health risks to you and your family.
CFR - Code of Federal Regulations Title 21 - FDA (3) Nutrition labeling shall be provided in the label or labeling of any food for which a health claim is made in accordance with § 101.9; for restaurant foods, ...
FDA Investigation into Potential Link between Certain Diets ... Between January 1, 2014 and April 30, 2019, the FDA received 524 reports of DCM (515 canine reports, 9 feline reports). Approximately 222 of these were reported between December 1, 2018 and April ...
Authorized Health Claims That Meet Significant Scientific Agreement Authorized Health Claims That Meet the Significant Scientific Agreement (SSA) Standard Authorized health claims in food labeling are claims that have been reviewed by FDA and are allowed on food...
The FDA's Definition of 'Healthy' Is Getting an Upgrade Under the FDA's proposal, manufacturers can claim a food or drink product is "healthy" so long as it contains a meaningful amount of food from at least one of the food groups or subgroups outlined in the Dietary Guidelines. These include vegetables, fruits, grains, dairy, and protein foods. "People eat food, not nutrients," Shelley ...
What You Need to Know About Health Claims on Food Labels and Dietary ... In general, health claims are statements made on food product labels or dietary supplements that boast some type of health benefit. This may seem simple, but the FDA doesn't treat every claim the same way. Label claims come in multiple forms: Health claims (which comprise of authorized health claims and qualified health claims)
FDA New "Healthy Food" Label Guidelines - healthsurgeon.com The FDA is establishing new rules for using the term "Healthy Food" on labels and packaging. The last labeling rules were established in 1994. That is 28 years ago, it is time for an update. With the implementation of the new rules foods must meet specific nutrient related criteria to make a "healthy" claim. According to the fda.gov:
Health Claims on Food Labels - LabelCalc Health claims, according to the FDA, are statements about the relationship between a food product or ingredient and a reduced risk of disease or a health condition. Basically, the FDA distinguishes two kinds of health claims: "authorized" and "qualified." Authorized Health Claims: Claims that have significant scientific agreement (SSA ...
Food Label Claims: What You Can and Can't Trust - WebMD The FDA is updating its definition for this claim. Until then, companies can make the "healthy" claim if the fats in their foods are mostly mono- and polyunsaturated fats. The healthy claim also...
Enforcement Policy Statement on Food Advertising The Federal Trade Commission (FTC) is issuing this statement to provide guidance regarding its enforcement policy with respect to the use of nutrient content and health claims in food advertising. The Commission believes the statement is appropriate in light of the passage of the Nutrition Labeling and Education Act of 1990 (NLEA), 1 and the ...
FDA Proposes to Update Definition for “Healthy” Claim on ... FDA Proposes to Update Definition for "Healthy" Claim on Food Labels Constituent Update September 28, 2022 The U.S. Food and Drug Administration today issued a proposed rule to update the...
FDA Proposes Updated Definition of 'Healthy' Claim on Food ... 28 Sept 2022 — The proposed rule would update the “healthy” claim definition to better account for how all the nutrients in various food groups contribute and ...
FDA Proposes New 'Healthy' Claim on Food Labels FDA Proposes New 'Healthy' Claim on Food Labels. Sept. 28, 2022. Its food group-based approach continues prohibitions but allows salmon and nuts to be considered healthy. Dave Fusaro. The FDA today (Sept. 28) issued a proposed rule to update the definition of the "healthy" claim on food & beverage packaging. Interested parties have ...
FDA perspectives on health claims for food labels - PubMed The U.S. Food and Drug Administration's regulatory authority over health claims was clarified in 1990 legislation known as the Nutrition Labeling and Education Act (NLEA). This law established mandatory nutrition labeling for most foods and placed restrictions on the use of food label claims charact …
Changes to the Nutrition Facts Label | FDA - U.S. Food and ... Mar 07, 2022 · Manufacturers with $10 million or more in annual sales were required to update their labels by January 1, 2020; manufacturers with less than $10 million in annual food sales were required to ...
Qualified Health Claims - FDA Food manufacturers can petition the agency to consider exercising enforcement discretion for the use of a qualified health claim. The FDA does not "approve" qualified health claim petitions.
Food Packaging Claims | American Heart Association It's important to understand what these claims mean so you can make informed decisions about the food you buy for yourself and your family. There are three categories of claims defined by statute and/or FDA regulations that can be used on food and dietary supplement labels: health claims, nutrient content claims, and structure/function claims.






:max_bytes(150000):strip_icc()/juicy-juice-no-sugar-400x400-b1fb04c46e9e4c8392ce8881614c021a.jpg)






















Post a Comment for "38 fda health claims on food labels"