40 fda approved health claims on food labels
Introduction to Food Product Claims — FDA Reader A Qualified Health Claim is a statement approved by the FDA for use on food labels that has strict wording requirements. When there is emerging evidence between a food and the reduced risk of a disease or health condition, but not enough for the FDA to issue an Authorized Health Claim, the FDA may approve a "Qualified Health Claim". The FDA Wants to Update the Definition for "Healthy" Claims on Food Labels The FDA is looking to regulate the use of the word because of the rise of diet-related illnesses in the U.S. "In the current marketplace, about 5 percent of all packaged foods are labeled as 'healthy,'" the FDA writes in the proposal. "Because nutrition science has evolved over time, updating the definition of the implied nutrient content claim ...
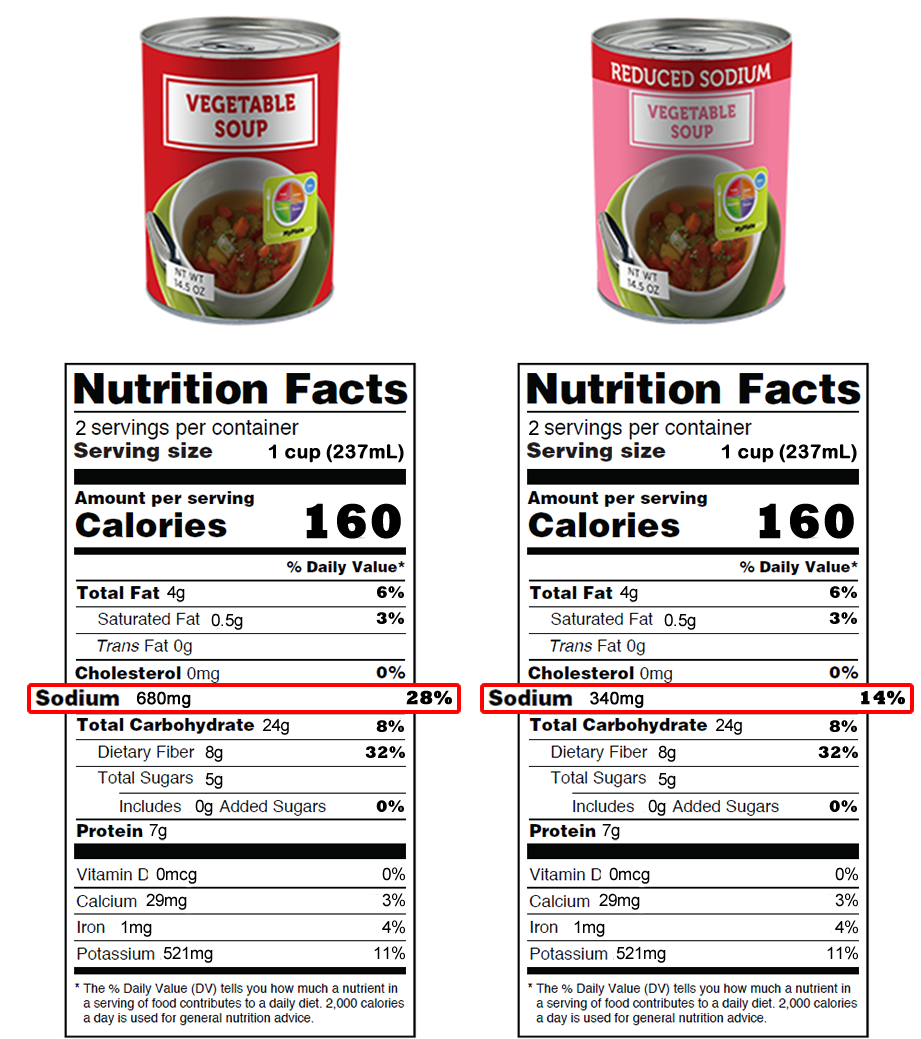
Food Packaging Claims | American Heart Association It's important to understand what these claims mean so you can make informed decisions about the food you buy for yourself and your family. There are three categories of claims defined by statute and/or FDA regulations that can be used on food and dietary supplement labels: health claims, nutrient content claims, and structure/function claims.

Fda approved health claims on food labels
Questions and Answers on Health Claims in Food Labeling | FDA All health claims, whether authorized or qualified, require pre-market review by the FDA. Under federal law, the FDA approves by regulation authorized health claims for use in food labeling only if... A Guide to FDA Regulation of Food Labeling Claims Among the FDA-regulated claims commonly declared on food labels are nutrient-content claims, health claims, qualified health claims and structure/function claims. Additionally, FDA has authority over claims related to gluten content, genetically modified organisms (GMOs) and "natural." Authorized Health Claims That Meet Significant Scientific Agreement Authorized Health Claims That Meet the Significant Scientific Agreement (SSA) Standard Authorized health claims in food labeling are claims that have been reviewed by FDA and are allowed on food...
Fda approved health claims on food labels. FDA proposes updates to 'healthy' claim on food packages | CNN The US Food and Drug Administration is proposing changes to the nutrition standards that foods must meet before they can carry the "healthy" label on their packages. The proposal comes as the ... FDA perspectives on health claims for food labels - PubMed This law established mandatory nutrition labeling for most foods and placed restrictions on the use of food label claims characterizing the levels or health benefits of nutrients in foods. NLEA set a high threshold for the scientific standard under which the U.S. Food and Drug Administration (FDA) may authorize health claims, this standard is ... FDA approves cardiovascular health claim on certain oil labels ... The FDA-approved health claim is as follows: ... Further, this oil label update comes as a part of the FDA's new strategy to modernize and prioritize health claims on food labels. They also aim to ... Health Claims on Food Labels - LabelCalc Health claims, according to the FDA, are statements about the relationship between a food product or ingredient and a reduced risk of disease or a health condition. Basically, the FDA distinguishes two kinds of health claims: "authorized" and "qualified." Authorized Health Claims: Claims that have significant scientific agreement (SSA ...
Authorized Health Claims That Meet Significant Scientific Agreement Authorized Health Claims That Meet the Significant Scientific Agreement (SSA) Standard Authorized health claims in food labeling are claims that have been reviewed by FDA and are allowed on food... A Guide to FDA Regulation of Food Labeling Claims Among the FDA-regulated claims commonly declared on food labels are nutrient-content claims, health claims, qualified health claims and structure/function claims. Additionally, FDA has authority over claims related to gluten content, genetically modified organisms (GMOs) and "natural." Questions and Answers on Health Claims in Food Labeling | FDA All health claims, whether authorized or qualified, require pre-market review by the FDA. Under federal law, the FDA approves by regulation authorized health claims for use in food labeling only if...





























Post a Comment for "40 fda approved health claims on food labels"