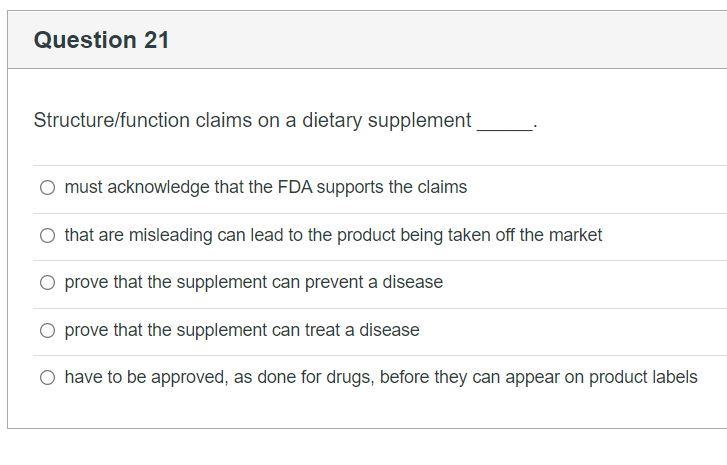
41 structure function claims on dietary supplement labels
''Structure/Function'' Claims in Dietary Supplement Labeling - ResearchGate Stephen H. McNamara Abstract Enactment of the Dietary Supplement Health and Education Act of 1994 (DSHEA) permitted manufacturers of dietary supplements to make structure/function'... How to achieve compliance in the USA? Dietary supplement labels The PDP must include the following mandatory information: 1. The Statement of Identify, for example Dietary Supplement 2. Net Quantity and Contents (which is the numerical count and product form for tablets / capsules) The Information Panel (IP) is defined as the panel of the box (or bottle) to the immediate right of the PDP.
FDA Labeling Guidance: Making Structure/Function Claims for Dietary ... The FDA permits supplements to so-called "structure-function claims," which are claims that describe the role of a nutrient or dietary supplement "intended to affect the structure or ...

Structure function claims on dietary supplement labels
› structurefunction-claimsStructure/Function Claims | FDA Mar 07, 2022 · Structure/Function Claims for dietary supplements ... appeared on the labels of conventional foods and dietary supplements as well as drugs. The Dietary Supplement Health and Education Act of 1994 ... Statements For Dietary Supplements: Structure/Function Claims Review ... The FDA published final regulations defining the types of structure or function claims permitted on dietary supplement labels in 2000, 21 CFR 101.93: Certain Types of Statements for Dietary ... Dietary Supplements: An Advertising Guide for Industry Application of FTC Law to Dietary Supplement Advertising A. Identifying Claims and Interpreting Ad Meaning 1. Identifying Express and Implied Claims 2. When to Disclose Qualifying Information 3. Clear and Prominent Disclosure B. Substantiating Claims 1. Overview 2. Ads that Refer to a Specific Level of Support 3. The Amount and Type of Evidence 4.
Structure function claims on dietary supplement labels. Labeling Guidance: Making Structure/Function Claims - Lexology The FDA permits supplements to so-called "structure-function claims," which are claims that describe the role of a nutrient or dietary supplement "intended to affect the structure or function in ... › food › dietary-supplements-guidanceDietary Supplement Labeling Guide: Chapter VI. Claims | FDA What health claims can be used on dietary supplement labels? ... What must I do when making structure/function claims in my products' labeling? You must (1) have substantiation that such statement ... › food › food-labeling-nutritionLabel Claims for Food & Dietary Supplements | FDA Among the claims that can be used on food and dietary supplement labels are three categories of claims that are defined by statute and/or FDA regulations: health claims, nutrient... PDF Permissible vs. Impermissible Structure/Function Claims for Dietary ... Structure/Function Claims for Dietary Supplements. 2 THE BASICS: ... the heart symbol on product label and labeling is an impermissible heart disease prevention claim.) 14 ... No more than thirty (30) days after a supplement bearing a structure/function claim is marketed, the manufacturer, packer, or distributor of the
Structure Function Claims - Dietary Supplement Experts Structure/function claims describe the role of a nutrient or dietary ingredient intended to affect the structure or function of humans, or characterize the documented mechanism (s) of action by which a nutrient or dietary ingredient acts to maintain such structure or function. Importantly, they cannot be disease claims. Dietary Supplements - Claims and Labeling Compliance for FDA/FTC Dietary supplements that make disease claims will be regulated by the FDA as drugs. Dietary supplements can make 'structure/function' claims (for example, 'calcium builds strong bones'). A structure/function claim describes the product's role in maintaining the 'structure or function of the body,' or 'general well-being.' Labeling rules are ... FDA Supplement Guide - innerself.com The 1994 Dietary Supplement Health and Education Act, which set up a new framework for FDA regulation of dietary supplements, requires consumers, as well as manufacturers, to be responsible for checking the safety of these products and determining the truthfulness of their label claims. Label Claims for Food and Dietary Supplement - LBS RCS.COM it contains criteria to determine when a labeling statement made about a dietary supplement constitutes a structure/function claim for which no prior fda review is required and when it constitutes a disease-related claim that requires either authorization of a health claim or review under the drug provisions of federal food, drug, and cosmetic …
Structure/Function Claims Basics - Dietary Supplement Experts Structure/function claims describe the role of a nutrient or dietary ingredient intended to affect the structure or function of humans, or characterize the documented mechanism(s) of action by which a nutrient or dietary ingredient acts to maintain such structure or function. Importantly, they cannot be disease claims. Constructing structure and function claims - Natural Products INSIDER Structure/function claims describe a nutrient's or dietary ingredient's effect on or maintenance of the structure or function of the body—for example, "calcium builds strong bones" or "antioxidants maintain cell integrity." A disease claim is an express or implied claim to diagnose, mitigate, treat, cure or prevent a disease—for ... FDA Labeling Guidance: Making Structure/Function Claims for Dietary ... the fda permits supplements to so-called "structure-function claims," which are claims that describe the role of a nutrient or dietary supplement "intended to affect the structure or function in humans" or characterize the "documented mechanism" by which the nutrient acts to maintain such structure or function, but do not claim to "diagnose, … › food › food-labeling-nutritionLabel Claims for Conventional Foods and Dietary Supplements Mar 07, 2022 · The Dietary Supplement Health and Education Act of 1994 (DSHEA) established some special regulatory requirements and procedures for using structure/function claims and two related types of dietary ...
Dietary Supplements Claims, Labels and Regulations | NSF Structure/function claims refer to the supplement's effect on the body's structure or function, including its overall effect on a person's well-being. Examples of structure/function claims include "Calcium builds strong bones" and "Antioxidants maintain cell integrity". Nutrient Content Claims
Challenge to Dietary Supplement Structure/Function Claims Preempted ... Posted on April 5, 2022. On March 31, 2022, the U.S. District Court for the District of Massachusetts held that Plaintiffs' claims— which alleged that the labels on three of Vitamin Shoppe, Inc.'s dietary supplements included false and misleading statements— were preempted by the Federal Food, Drug, and Cosmetic Act (the "Act").
Structure/Function Claim Notification for Dietary Supplements Office of Dietary Supplement Programs (HFS-810) Center for Food Safety and Applied Nutrition Food and Drug Administration 5001 Campus Drive College Park, MD 20740-3835 Contact the Office of...
Dietary Supplements: An Advertising Guide for Industry Application of FTC Law to Dietary Supplement Advertising A. Identifying Claims and Interpreting Ad Meaning 1. Identifying Express and Implied Claims 2. When to Disclose Qualifying Information 3. Clear and Prominent Disclosure B. Substantiating Claims 1. Overview 2. Ads that Refer to a Specific Level of Support 3. The Amount and Type of Evidence 4.
Statements For Dietary Supplements: Structure/Function Claims Review ... The FDA published final regulations defining the types of structure or function claims permitted on dietary supplement labels in 2000, 21 CFR 101.93: Certain Types of Statements for Dietary ...
› structurefunction-claimsStructure/Function Claims | FDA Mar 07, 2022 · Structure/Function Claims for dietary supplements ... appeared on the labels of conventional foods and dietary supplements as well as drugs. The Dietary Supplement Health and Education Act of 1994 ...

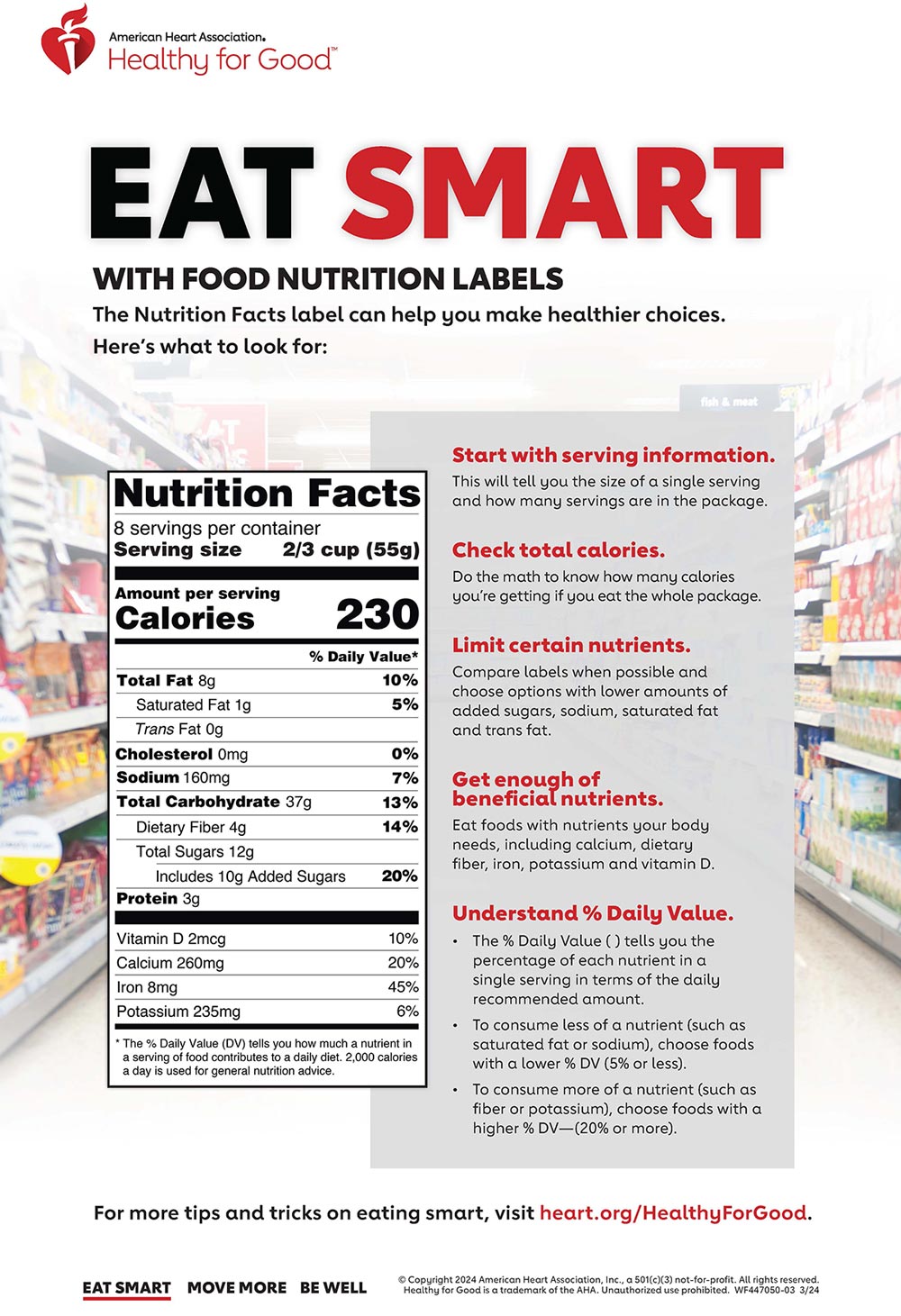



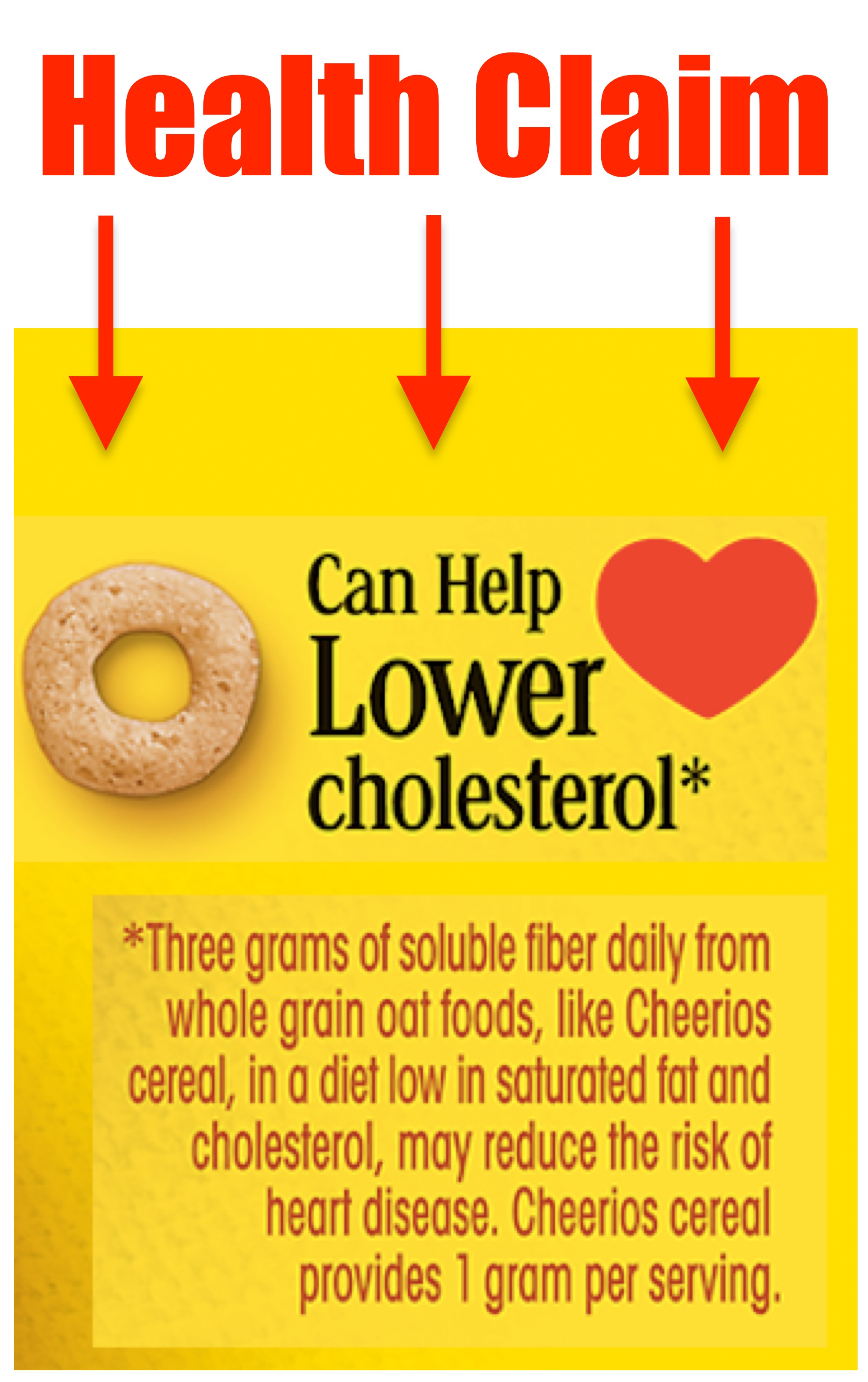







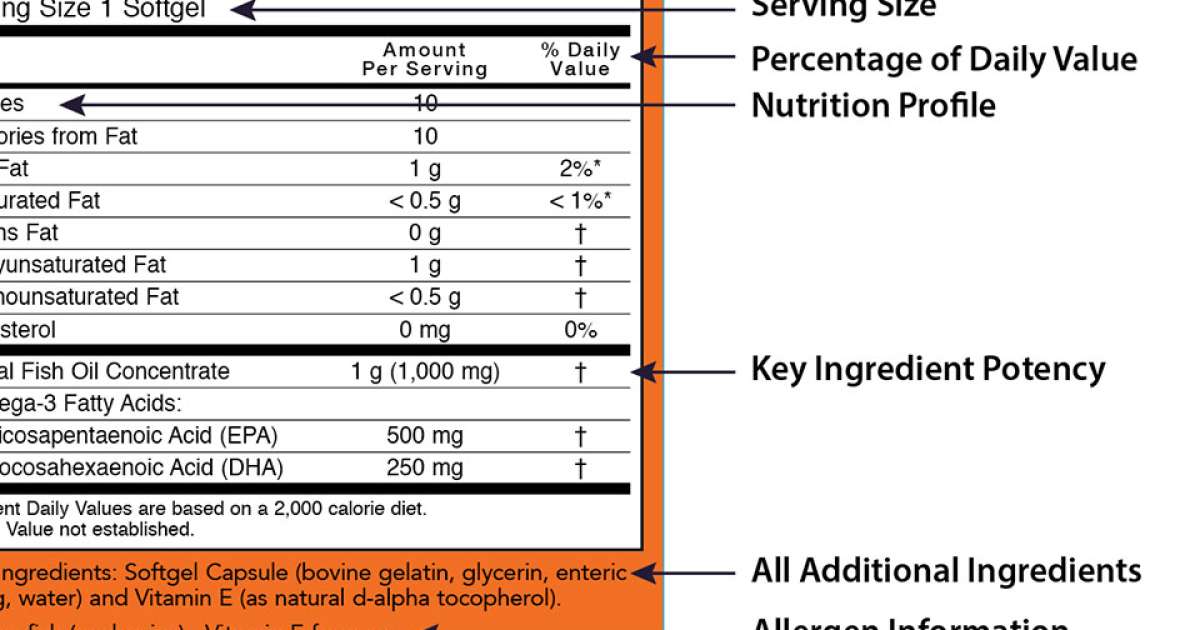





:no_upscale()/cdn.vox-cdn.com/uploads/chorus_asset/file/3652086/heart-check-label-crop.0.jpg)

:no_upscale()/cdn.vox-cdn.com/uploads/chorus_asset/file/3650624/quakerlabel-shelf.0.jpg)







Post a Comment for "41 structure function claims on dietary supplement labels"